Background:Blinatumomab(Blincyto) is a CD3-CD19 bispecific T-cell engager for treating B-cell acute lymphoblastic leukemia(B-ALL). Blincyto has been available in China since Aug 2021. Standard treatment duration of Blincyto is 28 days(d) per cycle. Due to economic reasons, part of the Chinese patients(pts) received 14d of infusion per cycle. Whether the 14-d regimen impact outcomes remains uncertain. To investigate the efficacy of 14-d regimen, we retrospectively analyzed the data of pts treated with Blincyto in our center.
Methods:From Sep 2021 to Jul 2023, all of the B-ALL pts treated with Blincyto in our center were enrolled. Minimal residual disease(MRD) was monitored by 8-color flow cytometry before, d14 and after each cycle of Blincyto. MRDneg was difined as below 10 -4. Different doses of Blincyto were given based on disease burden: relapsed or refractory(R/R) pts were given 9ug, d1-7, 28ug, d8-28/cycle; for complete remission(CR) pts with MRD positive(MRDpos) or MRD negative(MRDneg), the treatment duration varied from 14 to 28d with or without dose escalation per cycle, which was decided by physicians. Ph+ and ph-like pts were recommended to continue tyrosine kinase inhibitors treatment. Intrathecal chemotherapies were given for pts with central nervous system (CNS) involvement. Progression free survival(PFS) was defined as time from treatment initiation until progression, relapse, or death; overall survival(OS) was defined as time from treatment initiation until death.
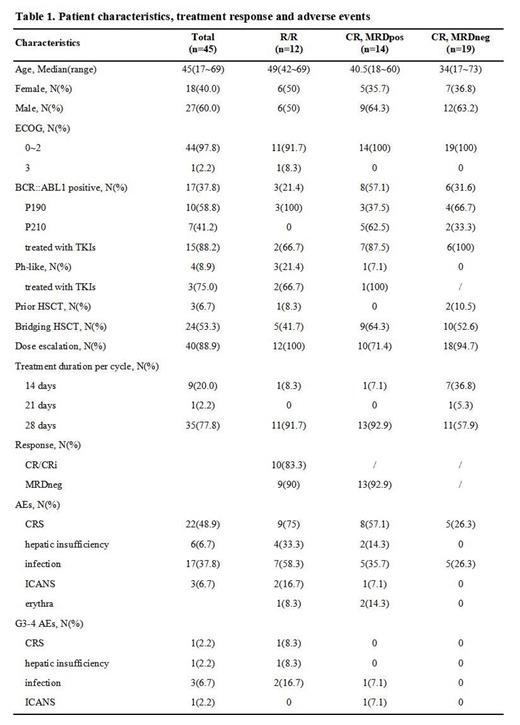
Results: From Sep 2021 to Jul 2023, a total of 45 pts were enrolled(12 R/R, 14 CR MRDpos, 19 CR MRDneg)(Table 1). The median age was 45 year(range, 17~69) and 27(60%) pts were male.
In 12 R/R pts(6 ph-, 3 ph-like, 3 ph+), the median disease burden before infusion was 28.9% (range, 6%~92.5%). After 1 cycle of Blincyto, 83.3%(10/12) pts achieved CR/CRi, 90% (9/10) achieved MRDneg. CR rate was 100% in 6 ph- pts, while MRDneg rate was 83.3%(5/6). Among 3 R/R ph-like pts, 2 pts achieved CR(66.7%), both MRDneg(100%). Same results were observed among 3 R/R ph+ pts. The ph+ nonresponder stopped infusion on d9 due to Grade(G) 2 immune effector cell associated neurotoxicity syndrome(ICANS), while the ph-like nonresponder was refractory to induction therapy and failed in subsequent salvages and died. No deaths was observed in other 11 pts. By Jul 2023, the median follow-up time was 8.6 months(m) and the estimated PFS was 13.4m.
Among 14 CR MRDpos(5 ph-, 1 ph-like, 8 ph+) pts, the median MRD level was 0.075% (range, 0.01%~3.58%). After 1 cycle of Blincyto infusion, MRDneg was achieved in 92.86% (13/14) pts, except 1 ph+ nonresponder who developed CD19 loss at d14. By Jul 2023, the median follow-up was 6.78m, estimated mPFS was 13.2m. Two pts died(1 Covid-19, 1 CNS involvement).
Blincyto was applied in 19 CR MRDneg pts post induction. The median treatment cycle was 1(range, 1-10). With a median follow-up of 4.73 months, PFS and OS were both 100%.
In this study, 2 refractory pts and 1 CR MRDpos pt(all ph-) received 14-d regimen. All of them achieved MRDneg(100.0%) after 1 cycle, suggesting that the 14-d cost saving regimen may be feasible. However, MRD-rising was observed just 1 month after response in a refractory pt who developed a CNS relapse after allogeneic transplantation(allo-HSCT). The relatively short MRDneg duration of the pt may due to an insufficient depth of response.
Among 14 CR MRDpos pts, the first 4 pts were treated without dose escalation, 75%(3/4) pts achieved MRDneg. The CRS rate was 100%(4/4)(G1-2) and a G3 ICANS(seizure) was observed in the fourth pt. In order to improve tolerance, the regimen was adjusted to apply dose escalation(9μg/d, 3~7d) in all 10 pts afterwards. No more ICANS was observed and the occurrence of CRS decreased to 40%(4/10)(G1-2). The MRDneg rate was 100%.
Pts eligible for transplantation all successfully bridged to allo-HSCT after Blincyto treatment(n=24), all of them obtained MRDneg before allo-HSCT. By Jul 2023, the median follow up time was 8.23 months, 3 pts relapsed post allo-HSCT(estimated PFS, 17.3m) and no deaths was observed.
Main AEs are listed as Table 1.
Conclusion: Blincyto is a safe and effective option for B-ALL pts with different disease burden. For CR MRDpos pts, the 14-d cost saving regimen may be effective but the depth of the response requires further study. Meanwhile, our results suggested that dose escalation may improve tolerance in CR MRDpos pts without impairing efficacy.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Blinatumomab (Blincyto) is a bispecific T-cell engager. It was approved for the treatment of relapsed/refractory and MRD-positive CR BCP-ALL patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal